FDA Authorizes Pfizer Booster for Kids 5 to 11, Bypasses Advisory Panel
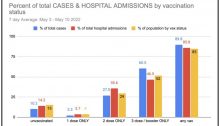
Megan Redshaw | 17 May 2022 The U.S. Food and Drug Administration today granted Emergency Use Authorization for Pfizer’s COVID-19 booster for children ages 5 to 11, without convening its vaccine advisory panel and despite the latest data showing higher case rates in vaccinated children compared with those not vaccinated. The U.S. Food and Drug…