JAMES LYONS-WEILER | 23 Feb 2023
It’s being reported as finding these events, but that they are rare. Relative to exactly what?
Let’s get right to the facts about the most recent study.
Some important facts about this study:
A. ARBITRARY SIGNIFICANCE LEVEL
Without justification, the authors adopted a lower bound confidence interval of LBCI > 1.5+0.95CI as a significance level cut-off for prioritized safety signals. LCBI is a conservative estimate of the potential risk associated with a treatment or intervention; this assumes the risk ratio distribution of all adverse events are identical and can conflate statistical significance with clinical significance. Other risk signals lower than LBCI > 1.5+0.95CI no doubt have clinical significance.
B. OBSERVED:EXPECTED RATIOS OF ADVERSE EVENTS WERE RELATIVE TO OTHER VACCINES, NOT RELATIVE TO UNVACCINATED
“Expected rates (of adverse events) were obtained by participating sites using pre-COVID-19 vaccination healthcare data stratified by age and sex. Observed rates were reported from the same healthcare datasets since COVID-19 vaccination program rollout. AESI occurring up to 42 days following vaccination with mRNA (BNT162b2 and mRNA-1273) and adenovirus-vector (ChAdOx1) vaccines were included in the primary analysis.”
C. ON “RATES OF NEUROLOGICAL CONDITIONS MUCH HIGHER FROM INFECTION THAN FROM VACCINATION”
The authors wrote: “Frontera et al. [46] concluded that chances of having a neurological event following acute SARS-CoV-2 infection were up to 617-fold higher than following COVID vaccination, suggesting that the benefits of vaccination substantially outweigh the risks.”
First, we should emphasize SEVERE. So no one should conclude “increased risks of neurological events following COVID-19 relative to vaccination”. But they will.
Second, here is that study – “Neurological Events Reported after COVID-19 Vaccines: An Analysis of Vaccine Adverse Event Reporting System” which actually reported “the rates of adverse neurological events reported after COVID vaccination were 132 to 617-fold lower”.
These rates are based on EXPECTED CASES. So how did they determined EXPECTED CASES following severe COVID-19?
“The expected number of events was calculated as (person-years x background rate)/100,000, where background rates were measured per 100,000 person years.”
So the expected rates of these outcomes for COVID-19 SEVERE INFECTION are based on population-level rate estimates in UNINFECTED and INFECTED PERSONS prior to COVID-19, which, of course, is also a variously vaccinated population (for any number of other vaccines).
Meanwhile the estimates for the VACCINE-RELATED EVENTS are based on … GSVD network report-based estimated SAEs for other vaccines prior the EUA of COVID-19 vaccines.
One of the main challenges is ensuring that the pre-vaccine data is truly comparable to the post-vaccine period data. Changes over time in population health, healthcare practices, or the introduction of other medical interventions can affect the comparability of the two datasets.
“Observed-to-expected ratios (O:E) were then calculated as the number of observed neurological cases divided by the number of expected cases.”
These were then “compared to studies that reported a composite incidence of all neurological events acutely after SARS-CoV-2 infection”,
“…the rates of adverse neurological events reported after COVID vaccination were 132 to 617-fold lower (O:E 0.002–0.008 with 105,214 observed cases compared to 13,908,927-64,941,552 expected cases.”
This actually demonstrates that increase detected due to infection relative to severe COVID-19 infection is due to low VAERS reporting.
D. Notice the study provides NO DATA ON UNVACCINATED RATES.
The only plausible conclusion that might be divined from this study is:
People who are vaccinated also get mild and severe COVID-19, yet the largest COVID-19 vaccine study nevertheless found:
6.1x more Myocarditis risk due to COVID19 vaccine relative to other vaccines
6.9x more Pericarditis risk due to COVID19 vaccine relative to other vaccines
3.8x more Encephalomyelitis risk due to COVID19 vaccine relative to other vaccines
2.5x more Guillain-Barre risk due to COVID19 vaccine relative to other vaccines
3.2x more Blood Clots risk due to COVID19 vaccine relative to other vaccines.
E. No Study of Vaccinated then Infected vs. Infected then Vaccinated vs. Infected Alone vs. Vaccinated Alone
A critical limitation in the current landscape of COVID-19 research is the absence of comprehensive studies comparing outcomes among four distinct groups: those vaccinated then infected, those infected then vaccinated, those infected alone, and those vaccinated alone. Such comparative analyses are crucial for understanding the nuanced effects of vaccination and infection sequences on immunity, disease severity, and long-term health outcomes.
Without these data, our grasp of adverse events is incomplete. This gap in research hinders the development of fully informed vaccination strategies and public health policies, potentially affecting the effectiveness of pandemic response efforts. Moreover, it leaves significant questions unanswered regarding adverse events following infection, following vaccination, and their interplay, which are essential for guiding decisions on booster doses and tailoring recommendations for different population segments.
F. NO WAY TO ESTIMATE NUMBERS OF EACH TYPE OF ADVERSE EVENT
The paper and the Supplementary Material provide all of the information need to estimate the actual numbers of each type of adverse events in the full population – except detailed observed event rates per vaccine type, gender etc.
The authors could easily share the count data.
G. What The Ethical Skeptic Said
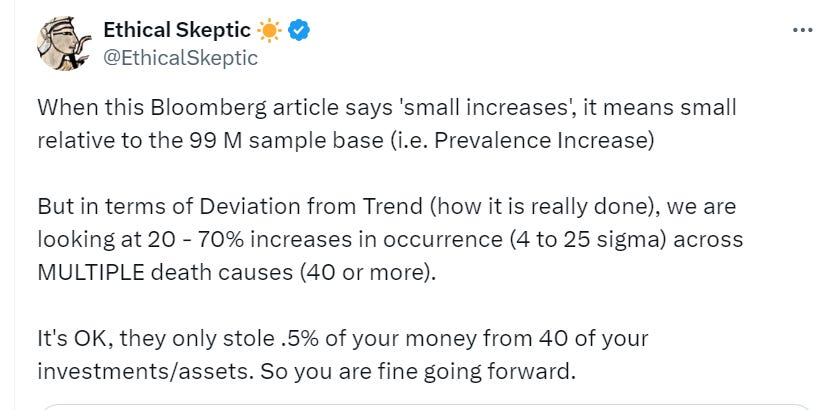
H. WHO ARE AUTHORS?
The first person listed as designing the study is Anders Hviid, who has a history of publishing unrealistic rates of events in studies given their population prevalence. See, for example:
An Autopsy on Hviid et al. 2019’s MMR/Vaccine Science-Like Activities – jameslyonsweiler.com
in which I revealed that Hviid’s study of autism in Denmark had a study-wide rate of ASD diagnosis of 0.9-1% over a time period when the prevalence of ASD in Denmark was 1.65%.
I. Conflicts of Interest ABOUND
Hviid alone lists 5 COIs, and since the US CDC provided funded, we can all assume the study is biased. Other conflicts include GSK, AbbVie Inc, Pfizer, Inc., Sanofi Inc. and many others:
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jeffrey C. Kwong reports financial support was provided by Centers for Disease Control and Prevention. Naveed Z. Janjua reports financial support was provided by Centers for Disease Control and Prevention. Anders Hviid reports financial support was provided by Global Vaccine Data Network. Helen Petousis-Harris reports financial support was provided by New Zealand Ministry of Health. Steven Black reports a relationship with GSK that includes: consulting or advisory. Jeffrey C. Kwong reports a relationship with Canadian Institutes of Health Research that includes: funding grants. Jeffrey C. Kwong reports a relationship with Public Health Agency of Canada that includes: funding grants. Naveed Z. Janjua reports a relationship with AbbVie Inc that includes: consulting or advisory and speaking and lecture fees. Naveed Z. Janjua reports a relationship with Gilead Sciences Inc that includes: speaking and lecture fees. Anders Hviid reports a relationship with Independent Research Fund Denmark that includes: funding grants. Anders Hviid reports a relationship with Lundbeck Foundation that includes: funding grants. Anders Hviid reports a relationship with Novo Nordisk Foundation that includes: funding grants. Anders Hviid reports a relationship with VAC4EU that includes: consulting or advisory. Finnish Institute for Health and Welfare (THL) conducts Public-Private Partnership with vaccine manufacturers and has received research funding from Sanofi Inc. Petteri Hovi has been an investigator in these studies, but has received no personal remuneration. Helen Petousis-Harris has served on expert advisory boards and had speaking engagements for Pfizer and GSK. She has also received research funding from GSK. She has not received any personal honoraria. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.


